advantage
Simultaneous detection of several different signals in a certain period of time
Comprehensive analysis of data using a variety of algorithms and curve fitting
Create a simple experimental solution using the workflow editor
Introduction
Simultaneous detection of the output of several different signals over a period of time is particularly helpful in studying the effects of proteins or compounds on cell growth or gene expression. In this application note, we use SoftMax® Pro version 7 software to simultaneously detect cell growth (light absorption) and protein expression (fluorescence).
In bacteria, the lac operon is a gene group regulated by a promoter encoding ß-galactosidase. This transformed plasmid is used to express the protein of interest by inserting a protein expression sequence into a plasmid containing the lac operon and transfecting the bacteria. Typically, the lac promoter is allosterically inhibited, but in the presence of isopropyl-ß-D-1-thiogalactoside (IPTG), the repressor is released from the promoter sequence causing the protein of interest. expression. Conversely, if the IPTG is excessive, the cells will be converted to preferential use of intracellular substances for protein expression and cell growth will be hindered.
By simultaneously measuring cell density and protein expression, we show how E. coli responds to serial dilutions of IPTG.

material
· SpectraMax® M2 Multi-Function Microplate Reader (Molecular Devices)
· Luria Broth (LB) Medium (Teknovacat.#L8080)
· Ampicillin (Teknova cat.#A9525)
· 1 M IPTG (Teknova cat. #I3431)
· 96-well transparent flat-bottom polystyrene multiwell plate (Greiner cat. #655-161)
· E. coli plasmid containing pBbE5-RFP (Keasling Lab)
method
E. coli containing the pBbE5-RFP plasmid (Fig. 1) was provided by Professor JayD. Keasling2. Escherichia coli (E. coli) strain was cultured in LB medium containing 100 μM ampicillin, and when the OD600 was 0.3, 100 μL of E. coli was transferred to a transparent 96-well plate. E. coli was treated with a two-fold serial dilution of IPTG at a starting concentration of 500 μM. 0 μM IPTG and media-only controls were also tested separately.
The microplates were incubated in a SpectraMax M2 multi-plate reader at 32 °C and kinetic assayed. Using the workflow editor of SoftMaxPro7 software, create a kinetic cycle that includes both light absorption and fluorescence detection methods. The readings are shocked for 10 seconds. The parameters are set as shown in Table 1. Cycle every 10 minutes for a total of 24 hours. The kinetic data of the two detection methods produced were analyzed using SoftMax Pro7 version software.

result
The kinetic curves of light absorption and fluorescence detection are shown in Figures 2 and 3, respectively. In Figure 2, increasing IPTG concentration leads to a decrease in OD600, which means an adverse effect on bacterial growth. In Figure 3, increasing the IPTG concentration did not cause an increase in the amount of red fluorescent protein (RFP) expression. Conversely, increasing the concentration of IPTG results in a decrease in the amount of total RFP expression. This reduction may be due to stagnant bacterial growth.
In Figure 4, the light absorption and fluorescence data are processed to calculate the area under the curve (AUC). The software of SoftMaxPro7 was used to analyze and summarize the effects of IPTG on cell growth and protein expression. The effect of IPTG on fluorescence was similar in all concentrations except for the concentration of 500 μM, ie increasing the concentration of IPTG resulted in a decrease in bacterial growth (light absorption).
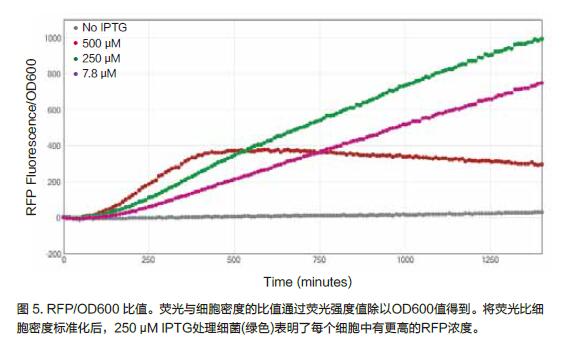
Calculating the ratio of RFP/OD600 can be used to determine protein expression relative to bacterial population density. Using the SoftMaxPro7 version software, we calculated the RFP/OD600 ratio (Figure 5). Based on kinetic tracking, 250 μM IPTG-treated bacteria expressed the most RFP per cell.



in conclusion
We show that the Molecular Devices SoftMaxPro7 version of the software and multi-function microplate reader can simultaneously detect many biological events over a period of time. Although the experiments shown are simple, this application is also suitable for more complex experiments.
references
1. Malakar, P. and Venkatesh, KV (2012) Effect of substrate and IPTG concentrationson the burden to growth of Escherichiacoli on glycerol due to the expression of Lac proteins. Applied Microbiology and Biotechnology, 93(6), 2543-2549.
2. Lee, TS, Krupa, RA, Zhang, F., Hajimorad, M., Holtz, WJ, Prasad, N., Lee, SK, and Keasling, JD (2011) BglBrick vectors and datasheets: asynthetic biology platform for geneexpression Journal of Biological Engineering, 5, 12.
Garden Supply Store,Indoor Gardening Supplies,Landscape Supplies,Magic Garden Supplies
Changzhou Satidi Import and Export Co., Ltd. , https://www.czguanjiechuck.com