Medical Network February 1st, "AI drug research and development" is not a new concept. As early as 1981, the magazine "Discovery" described "computers are expected to improve the efficiency of screening of medicinal molecules. Chemists no longer have to stay in the lab all week, all month, to test those molecules that computers find difficult to succeed."
In recent years, following the basic formation of the AI ​​medical imaging market, China's AI drug research and development companies have begun to enter the stage from scratch. Especially in 2018, with the entry of more players, capital will follow.
From a global perspective, nearly 100 companies have explored the “AI+ new drug research and developmentâ€. In 2018, Scientific American and the World Economic Forum released ten emerging technologies, and artificial intelligence-assisted chemical molecular design-machine learning algorithms accelerated the development of new drugs.
Some researches predict that "AI+ new drug development" will be a new vent in the future.
In the development of new drugs, AI is no less inferior to humans.
Behind the high hopes of AI, the most common saying is that the development of new drugs can't escape the "double ten fate": it takes 10 years and costs 1 billion dollars.
According to Nature, the average cost of new drug development is as high as $2.6 billion, and up to 90% of projects will fail in the meantime.
In other words, “high cost, long cycle, and low success rate†are three thresholds for new drug development.
The development of new drugs involves four stages: drug discovery, preclinical research, clinical research, and approval. In the normal mode, for potential drug targets, researchers typically use high-throughput screening to screen millions of compounds. According to PhRMA statistics from the American Association of Pharmaceutical Research and Manufacturers, at the drug development stage, as many as 5,000 to 10,000 compounds were screened, but only 250 were able to enter preclinical studies, and only five were in the clinical research phase.

Image from the network
In addition, researchers in the process need to face a large amount of literature , translation, report writing, data entry, etc., which is complicated and cumbersome, and takes a lot of time and effort. McKinsey Chilukuri has said that based on a 10-year cycle of drug development, revenues will not appear until 10 to 15 years later.
However, in recent years, the high return on recognition of new drug research is not ideal. The data shows that in 2017, the global TOP12 pharmaceutical giant's return on investment in research and development was only 3.2%, the lowest level in eight years. In addition, there are fewer and fewer late stage projects in the global new drug pipeline.
Therefore, breaking through the limitations of the human brain's understanding of biology, introducing AI technology, mining and screening compounds from a large number of data, and accurately predicting the physical and chemical properties, drug properties and toxicity risks, is expected to improve the speed and efficiency of new drug development. .
Some analysts believe that in the medium term, AI's value growth in the pharmaceutical industry may be equivalent to a 5% to 10% increase in sales.
In April 2018, a report in Nature showed that the University of Shanghai professor Mark Waller used reverse search analysis and path prediction using three search methods similar to the Alphago algorithm. The results show that this method performs well in double-blind tests, and the prediction results are not inferior to human experts.
   The exploration of "AI+ new drug research and development"
For different aspects of new drug development, AI technology can be used for target discovery, new drug synthesis route design, drug effectiveness and safety prediction, crystal form prediction, drug molecular design and other scenarios.
Globally, Atomwise offers drug candidate prediction services that have been used to simulate two compounds for Ebola treatment in a single week; Benevolent AI applies AI to medical research databases to quickly screen and organize data, and has Get a certain number of new drugs in the clinical phase.
BenevolentBio used JACS technology to identify 100 potential compounds for the treatment of amyotrophic lateral sclerosis (ALS) and successfully screened 5 compounds; BergHealth screened up to 250,000 disease tissue samples for early cancer New biological indicators and biomarkers, etc.
In China, Jingtai Technology is an early pioneer of “AI+ New Drug Developmentâ€. It mainly applies AI technology to early drug screening, drug design, drug repositioning and drug redirection in the process of drug discovery to improve the efficiency of drug development and reduce risk.
By 2018, in-depth wisdom, zero-tech, intellectual medicine technology, cloud software, etc. have also appeared in the field of vision, from time to time to send financing news.
Overall, there have been no successful cases of global AI drug research and development, but foreign related companies have used new drugs developed by AI to enter clinical phase II. Domestically, after the completion of the “AI+ New Drug Development†from 0 to 1, it also opened the 2.0 era.
On the other hand, the exploration of “AI+ new drug research and development†also means cooperation between IT companies and pharmaceutical companies.
“R&D outsourcing†is one of the cooperation models. In this model, AI companies model and screen candidate drugs based on data and target information provided by pharmaceutical companies. For pharmaceutical companies, this mode of operation is relatively lightweight, but because medical data is extremely sensitive, it is often accompanied by many conditions to ensure data security.
Correspondingly, in recent years, there have also been many hand-to-hand cooperation between large pharmaceutical companies and AI intelligent enterprises .
In 2015, pharmaceutical giant Merck and A AI company Atomwise reached a cooperation, the cooperation mainly involved drug efficacy and safety prediction; in 2016, Johnson & Johnson held hands with BenevolentAI to carry out drug excavation of small molecule compounds still in the experimental stage; 2017, competition Nofi and Exscientia sign a potential license of 250 million euros to develop bispecific small molecule drugs for metabolic diseases; in 2018, WuXi PharmaTech and Insilico Medicine work together on preclinical targets for challenging biological targets Drug candidate molecule development and more.
   "AI+ new drug development" will be a long-term battle
While pharmaceutical companies are actively exploring AI, there are still many challenges that must be faced. After all, AI is only one of the auxiliary technical means for the development of new drugs, and it does not mean the only and omnipotent.
According to a survey conducted by BenchSci, 41% of the 330 drug development scientists do not understand AI technology and cannot use AI to screen new drugs. In other words, the establishment of the corresponding AI talent training system will become an important foundation.
On the other hand, Thomas Chittenden, the head of the AI ​​project, has analyzed that the evaluation of new drug development and the effectiveness of its clinical trials is still an extremely complex recognition mode for artificial intelligence recognition systems.
In addition, the lack of high-quality drug targets and poor clinical conversion of animal models are also important factors affecting the maximum benefit of AI technology application.
Dr. Bruce Booth, an expert in artificial intelligence, shared an interesting phenomenon: Although computer design new drugs have existed for decades, the R&D output rate of the pharmaceutical industry has not increased, but has been decreasing year by year. The drug discovery time has not been shortened, and the cost has also been reduced. Lowered lower.
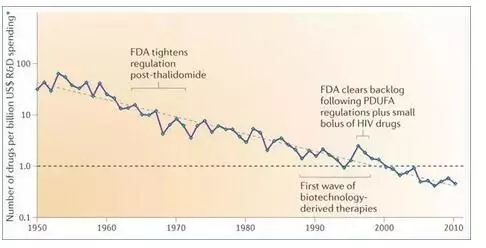
  The research and development output rate of new drugs has been declining for decades.
What it means is that AI has not brought a big change to the development of new drugs. Therefore, in the long run, applications such as AI technology can minimize the failure rate, save drug development costs, and shorten drug development time.
However, it is undeniable that “AI+ New Drug Development†is still in its infancy and will continue for a long time at this stage. At present, although the value of AI technology for different stages of new drug research and development has been clear, it is at least 5-10 years for the overall development of AI-driven new drug research and development.
Mobile DVR is crucial for both personal and commercial vehicles. It not only helps drivers by eliminating blind spots but also provides recorded footage that can serve as evidence in the event of accidents or insurance claims. Additionally, many clients utilize Mobile DVR solutions for efficient fleet tracking management across various types of vehicles. This solution is a perfect way to save costs and time.
Mobile Dvr,Sd Card Mobile Dvr,Vehicles Mobile Dvr,Mobile Surveillance Dvr
Vsstech Co.,Ltd. , https://www.vsstechcctv.com