Qiao Lirui 1 , Rob Lewis 2 , Alex Hooper 2 , James Morphet 2 , Tan Xiaojie 1 , Kate Yu 3
1. Waters Technology (Shanghai) Co., Ltd., Shanghai, China
2. Waters Corporation Mass Spectrometry Technology Center, Manchester, UK
3. Waters Corporation, Milford, MA, USA
This application note describes how to quickly and accurately characterize the similarities and differences of chemical constituents of Shuanghuanglian oral liquid from two different manufacturers using the binary comparison workflow in the Waters® UNIFI® Natural Product Total Solution. Understanding the differences in composition between different manufacturers of the same product also helps the same manufacturer to monitor the quality stability of products between batches. A simple and effective solution is proposed for the work of binary comparison of all similar different samples.
Key words:
Binary comparison of traditional Chinese medicine products, UNIFI Chinese medicine database, UPLC®/QTof MS, UNIFI natural product solutions, UNIFI natural product workflow, Shuanghuanglian oral liquid, quality control of traditional Chinese medicine, product batch comparison
Background introduction:
Traditional Chinese medicine is both complex and magical, and has been developed for thousands of years. However, for a long time, how to judge the true, false, excellent and inferior quality of traditional Chinese medicine lacks systematic and scientific analysis methods. For example, the control of the active ingredients of Chinese medicinal materials in different producing areas has not been consistently and effectively applied to different cultivation methods, different harvesting seasons or the quality evaluation of Chinese medicinal materials after different processing techniques. In recent years, pharmacy workers have done a lot of work in the research of Chinese medicine quality standards, and accumulated a lot of valuable experience and methods. However, in general, the binary comparison of traditional Chinese medicine is time-consuming and costly. Some methods lack a certain scientific basis and are not sufficient and objective. This application note uses advanced separation detection technology combined with the new UNIFI natural product overall solution to solve the problem effectively.
The similarities and differences of chemical composition of different origins of medicinal materials, the difference of the same products of different manufacturers, the quality stability of products between different batches of the same manufacturer, etc., need to compare similar samples, observe the similarities and differences of components, and then find out the differences, and provide for later research. Clear direction. With the continuous evolution of analytical instrument technology, the use of high-resolution LC/MS instruments (such as UPLC/QTof MS) ​​is gradually being promoted. The high-resolution LC/MS instrument can shorten the analysis time of the sample and improve the separation efficiency. At the same time, it has the characteristics of adaptability, high specificity, low detection limit, small injection volume, low solvent consumption, high automation and high qualitative ability. The status of quality control in traditional Chinese medicine will also become higher and higher.
This application note uses the comparison of Shuanghuanglian oral liquid products from two different manufacturers as an application example to explain the latest Waters UNIFI natural product overall solution. The solution is based on ultra-high performance liquid chromatography (ACQUITY UPLC® I-Class), quadrupole time-of-flight mass spectrometry (Xevo® G2-S QTof MS) ​​and UNIFI Chinese medicine database, using a UNIFI binary comparison workflow for similar Samples are compared to quickly identify differences and provide a basis for later quality monitoring and development of relevant quality standards, which greatly increases work efficiency and reduces the operator's technical background.
The main sources of Shuanghuanglian oral liquid are honeysuckle, scutellaria and forsythia, which have the effect of dispelling wind and clearing the heat and detoxifying. It has certain curative effect on bacterial and viral infectious diseases such as upper respiratory tract infection, tonsillitis, pharyngitis and viral pneumonia. Among them, baicalin, forsythin and wogonin are the main active ingredients. There are many manufacturers in China that produce this oral liquid product. Therefore, we use this very representative example to illustrate the workflow of binary comparison in the overall solution of UNIFI natural products (Figure 1).
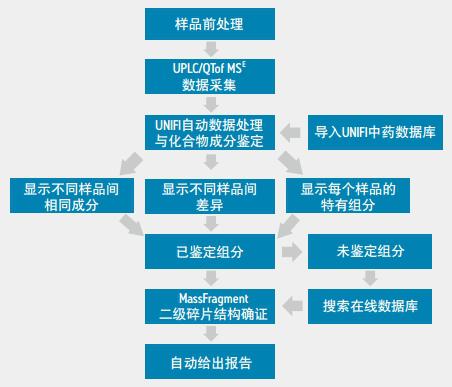
Figure 1 The workflow of binary comparison in the UNIFI natural product overall solution
Experimental conditions :
Sample processing:
Take 200 μl of Shuanghuanglian Oral Solution dissolved in 2 mL of H2O (equivalent to ten times dilution) and set aside. The injection volume is 1 μl.
Liquid chromatography conditions:
Instruments: ACQUITY UPLC I-Class with FTN Sample Manger
Column: ACQUITY UPLC HSS T3, 2.1 x 100 mm 1.8 μm, 40 ° C Sample chamber temperature: 15 ° C Mobile phase A: Water (0.1% formic acid)
Mobile phase B: acetonitrile
gradient:
time | Flow rate (ml/min) | Solvent A (%) | Solvent B (%) | curve |
0 | 0.5 | 95 | 5 | Start |
1 | 0.5 | 90 | 10 | 6 |
8 | 0.5 | 55 | 45 | 6 |
12 | 0.5 | 45 | 55 | 6 |
13 | 0.5 | 5 | 95 | 6 |
15 | 0.5 | 95 | 5 | 1 |
Mass spectrometry conditions:
Apparatus: Xevo G2-S QTof MS
Collection quality range: 100-1500 Da
Scanning time: 0.1 S
Acquisition mode: ESI+ and ESI-resolution mode, MS E
Lock mass: Leucine enkephalin (LE) 1 ppm (0.3 S scan, interval: 15 S)
Capillary voltage: 3 KV (ESI+) / 2.5 KV (ESI-)
Taper hole voltage: 100V
Collision energy (eV): low CE: 6 /High CE: 20-45
Ionization source temperature: 120 ° C Desolvation temperature: 500 ° C cone gas flow rate: 60 L / h
Desolvent gas flow rate: 1000 L/h
Data collection time: 15 min
result:
The data of the relevant chemical constituents of Shuanghuanglian oral liquid of two different manufacturers were collected by ultra performance liquid chromatography and quadrupole time-of-flight mass spectrometry. The binary comparison workflow in the UNIFI natural product overall solution was combined with the UNIFI Chinese medicine database for data processing. The results clearly showed the same component map, the difference component map and the unique component list of each sample in the two samples. The results of binary comparison between the two manufacturers showed that the content of forsythiaside A, forsythiaside B, D-hydroxyl foramen A, burdock, rutin, etc. in the oral solution of the manufacturer was much higher than that of the manufacturer. Oral liquid, while the content of baicalin in the second oral solution of the manufacturer is higher.
discuss:
The UNIFI natural product overall solution includes ACQUITY UPLC I-Class Ultra Performance Liquid Chromatography, Xevo G2-S QTof Quadrupole Time-of-Flight Mass Spectrometry and UNIFI software platform with Chinese medicine database. The solution includes five preset binary comparison workflow templates and a results report template that automates data collection and processing, database retrieval, structure validation, and print reporting.
Figure 2 is a comparison diagram of UPLC/QTof MS base peak ion chromatogram (BPI) of Shuanghuanglian oral solution of different manufacturers. This figure shows the advantages of using ultra-high performance liquid chromatography to analyze complex Chinese medicine systems with short run times (effective separation time of 12 min) and high separation efficiency and peak capacity. At the same time, quadrupole time-of-flight mass spectrometry provides mass spectral data with accurate mass information. In addition, it can be seen from Fig. 2 that not only the chemical composition of these two manufacturers is complicated, but also the composition of the oral liquid is mostly similar. If you do not use the help of effective software, you can quickly and accurately find two samples with such complex components. It is almost impossible to make commonalities and differences.
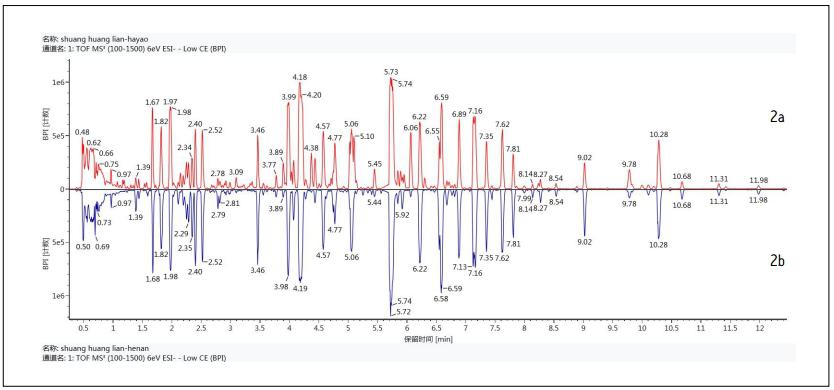
Figure 2 UPLC/QTof MS base peak BPI ion chromatogram binary comparison of Shuanghuanglian oral solution (2a: manufacturer one product; 2b: manufacturer two product).
Natural product studies Whether it is a single sample component or a comparison of the components of a different sample, the details of the component are the basis for further research. The workflow for the analysis of the composition of known medicinal samples has been elaborated in the previous application note "Analysis of components in green tea extracts using the UNIFI natural product overall solution" (literature number zh). In accordance with this workflow, we imported all the chemical components related to honeysuckle, astragalus and forsythia Chinese herbal medicines from the UNIFI Chinese medicine database, and then processed the data and identified the component information in the samples. Click on any of the preset binary comparison templates to get the result information associated with it. For example, Figure 3 shows the result information obtained by clicking on the Binary-Overview Plot.
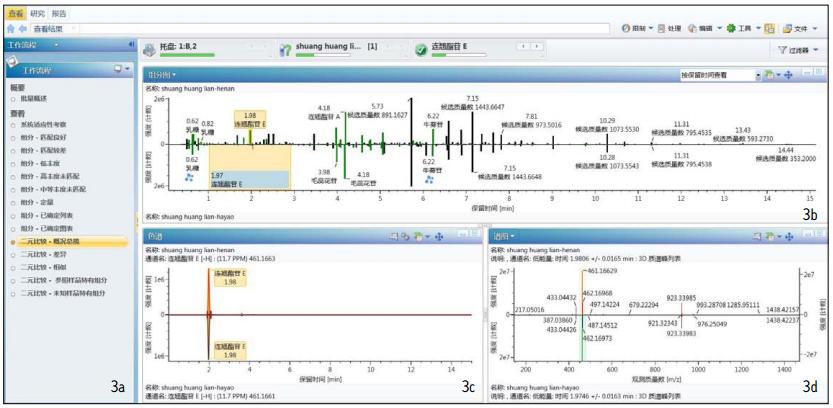
Figure 3 UNIFI software treatment of different manufacturers Shuanghuanglian oral liquid binary comparison results
Figure 3a shows the fourteen workflow templates preset in UNIFI. Figure 3b is a binary comparison of UPLC/QTof components for two factory samples. The green bar graph in the figure is the component that matches the components listed in the Chinese medicine database and is automatically identified by the software. The black bar graph is the component that cannot be automatically identified without matching. Figure 3c is a single selected ion chromatogram (XIC) comparison corresponding to Figure 3b (this figure shows a comparison of XIC of forsythiaside B with a retention time of 4.03 minutes). Figure 3d is a comparison of the low energy channel mass spectra corresponding to the components shown in 3c.
If you want to know more about the similarities and differences between the two samples, you can directly see the same components and different components between the two samples by clicking the preset Binary-Common working template or the Binary-Difference working template. For example, Figure 4 shows the results of clicking the Binary-Common work template. Figure 4a lists the same components in both samples (the initial result is 1978). Figure 4b shows the mass spectral response values ​​for each of the components corresponding to Figure 4a in two samples, reflecting the relative amount of this component in each sample (this figure shows baicalein). If you click on the Binary-Difference work template, you will get a similar interface. The result shows the difference in composition between the two samples (the initial result is 408).
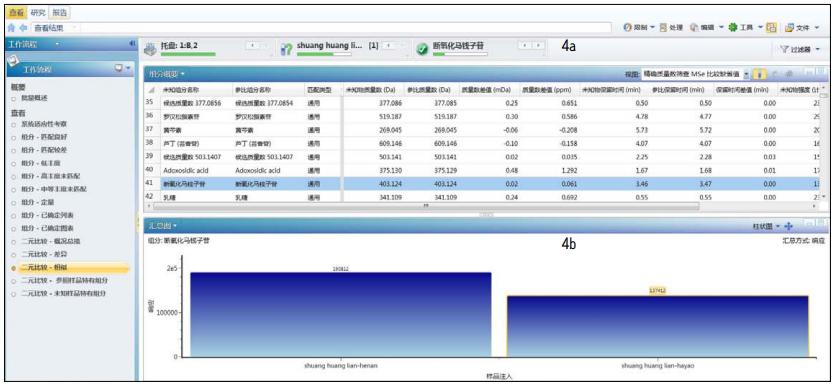
Figure 4 Results of clicking the Binary-Common work template
The similarities and differences between the two samples obtained by using the Binary-Common working template and the Binary-Difference working template can also be more visually displayed in the form of a bar graph. For example, after clicking on the Binary-Difference working template to get the difference in the composition of the two samples, you can directly see the map by clicking on the component map in the component summary list (Figure 5). This figure allows the staff to clearly and intuitively observe the difference between the two samples.
The difference between the two oral liquids showed that the content of baicalin in the manufacturer's sample was significantly higher than that in the manufacturer, and many components in forsythia such as forsythiaside A, forsythiaside B, and d-hydroxyl forsythiaside The content of A and other components in the manufacturer 2 is much higher than that of the manufacturer. It can also be seen directly from Figure 5 that some of the components in the sample did not match the ingredients listed in the Chinese medicine database, and thus no relevant identification results were obtained. At this time, manual identification can be further performed if necessary.
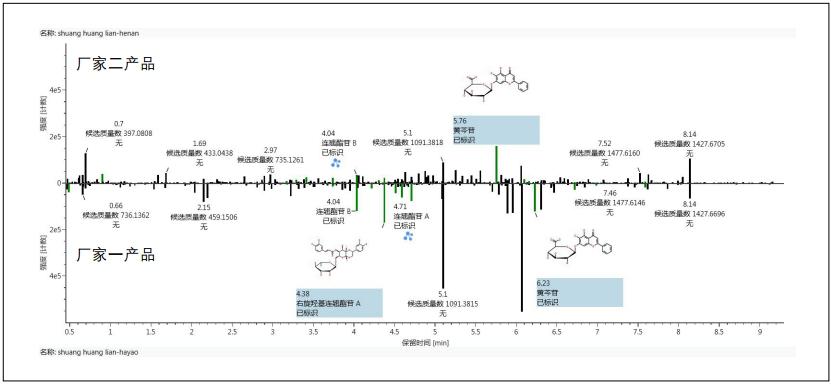
Figure 5 Differences in composition obtained after binary comparison of two oral liquid products
The details of the compositional differences between the two samples can be further understood by applying the two working templates Binary-Reference Unique and Binary-unknown Unique, so that a list of unique components in each sample can be obtained. The UNIFI binary comparison requires one of the samples to be listed as a reference sample (the chromatogram or mass spectrum peak is on) and the other sample as an unknown sample (the chromatogram or mass spectrum peak is below). In our sample, we randomly set the sample of the manufacturer 2 as the reference sample, and the sample of the manufacturer one is the unknown sample. If you want to further observe the unique components of a manufacturer's sample, simply click on the Binary-Unknown Unique template, as shown in Figure 6. The results suggest that the manufacturer has a unique composition of 193 components.
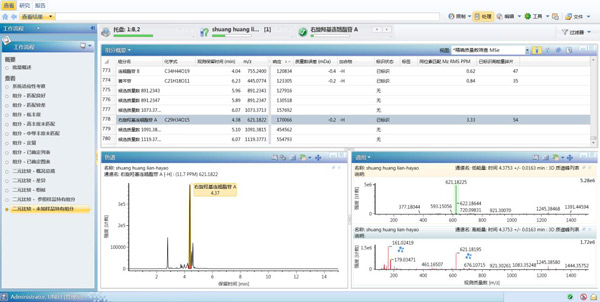
Figure 6 Click on the Binary-Unknown Unique template to obtain a unique component from the manufacturer's sample.
After the results are confirmed to be satisfactory, the report can be printed directly using the preset binary comparison report template. The report includes sample information, sample collection and post-processing methods, and a detailed list of the same components and different component information. Figure 7 shows a portion of the report showing the binary comparison of the two manufacturers, and the peaks are automatically labeled with the name of the compound. The results are clear at a glance.
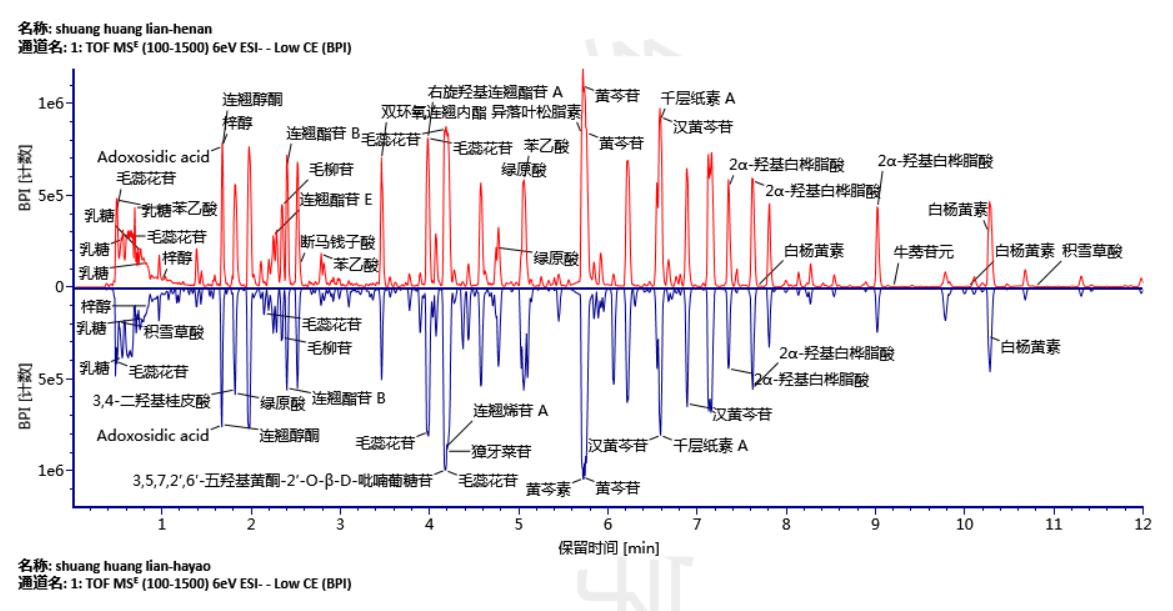
Figure 7 Report automatically obtained by importing natural product component information and summarizing the binary comparison report model
in conclusion:
This application note describes how to compare the composition and similarities of two different manufacturers Shuanghuanglian oral liquid using the binary comparison workflow in the UNIFI natural product overall solution. The workflow is based on UPLC/QTof MS data collection, supplemented by the Chinese medicine database and combined with the automatic identification function in UNIFI, which is a brand-new solution. This program is suitable for solving a series of problems, such as comparing different picking time and processing process of medicinal materials or the same medicinal materials from different origins, comparison of different manufacturers of the same product, quality stability of products between different batches of the same manufacturer, and the like. The solution contains a preset five binary comparison workflow templates and a report template associated with it, allowing researchers to quickly compare different samples and see the similarities and differences of their components, providing clear ideas for later research. In the end, the effectiveness and efficiency of traditional Chinese medicine research and quality monitoring have been greatly improved.
To download a clear app, please click: http://?cid=511436&lid=134778856&locale=zh_CN
Canned Bigeye Tuna,Bigeye Tuna Chunk in Oil Can,Tuna Salad Can
Zhejiang ocean family co.,ltd , https://www.ocean-family.com