Gene therapy for Alzheimer's disease, Johnson & Johnson reached a series of cooperation
January 16, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Johnson & Johnson Innovation and Janssen Pharmaceuticals, Inc. (JPI) signed an exclusive collaborative research agreement with the Gene therapy project team at the University of Pennsylvania. The collaboration will be conducted using both the adeno-associated virus (AAV) vector developed by the University of Pennsylvania and the antibody against Alzheimer's disease developed by JPI.

This collaboration aims to use AAV virus delivery to express therapeutic antibodies that target the major pathological markers of Alzheimer's disease. The use of AAV as a gene delivery method has the potential to alter the treatment of Alzheimer's disease and other brain diseases by biological therapies, opening up new ways to treat many destructive neurological diseases. Under this cooperation agreement, JPI will have the exclusive commercialization of the products developed worldwide.

â–² Dr. Paul Stoffels, Executive Vice President and Chief Scientific Officer of Johnson & Johnson (Source: Johnson & Johnson Official Website)
Dr. Paul Stoffels, Executive Vice President and Chief Scientific Officer of Johnson & Johnson, said: "Our priority is to improve the health of people around the world. This collaboration is a unique opportunity to explore new therapies. It works with companies, academic centers and public institutions. The revolutionary innovation in the healthcare industry is a step closer to solving many of the most pressing global medical problems."
In addition, Johnson & Johnson has some cooperative research worthy of attention, listed as follows:

Obesity is a growing epidemic that affects more than a third of adults in the United States and often causes diabetes, heart disease, stroke and certain types of cancer. Janssen Pharmaceuticals has established a multi-year research partnership with the G-Protein Coupled Receptor (GCPR) drug discovery incubator Beacon Discovery to research and develop a new generation of therapeutic drugs for the treatment of obesity and other metabolic diseases.
Johnson & Johnson and Janssen Research & Development have established an exclusive collaborative research agreement with Holobiome to develop microbial therapeutics for the treatment of central nervous system and enteric nervous system diseases. The collaboration will examine a bacterial group that can be used to create differentiated probiotic or over-the-counter products to address insomnia. In addition to sleep disorders, these bacteria can also be used to treat other underlying conditions.
Johnson & Johnson has partnered with Dermala in San Diego to develop microbial-derived skin treatments. Dermala's technical advantage lies in the use of the beneficial functions of skin bacteria to eliminate undesirable bacteria and balance the microbiota.
Psoriasis is a chronic immune-mediated disease affecting millions of people worldwide. Johnson & Johnson has expanded its research collaboration with Monash University to further explore potential triggers for psoriasis to discover and develop potential new therapies to prevent disease.
Johnson & Johnson Asia Pacific has partnered with the Institute of Industrial Technology to establish a co-financing agreement for lung cancer, chronic obstructive pulmonary disease, diabetes, eye health and digital health programs. The project will provide funding and guidance for one or more research projects for public sector participants and entrepreneurial companies.
Reference materials:
[1] J&J does Alzheimer's gene therapy deal, along with 14 other new collaborations
[2] Johnson & Johnson's official website
INTENDED USE
The One Step HCG Pregnancy Rapid Test Kit is a rapid
chromatographic immunoassay for the qualitative detection ofhuman chorionic gonadotropin (HCG) in urine to aid in the early
detection of pregnancy.
PRINCIPLE
The test utilizes antibodies including a
monoclonal HCG-β antibody and goat anti-mouse IgG on thenitrocellulose membrane with colloidal gold marked anti-HCG-α
monoclonal antibody as an mark tracer. The reagent is used to
detect the HCG in urine according to the principle of double
antibody sandwich method and gold immunochromatography
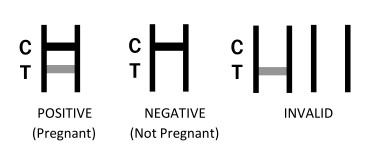
assay
There is a control line (C) controlling the reaction process shown
on the coated film. Based on test line`s (T) appearance to
determine whether the tested sample contains HCG (Human
Chorionic Gonadotrophin) or not.
MAIN COMPONENTS
Basic components: Sample pad, colloidal gold marked pad,
nitrocellulose membrane, absorbent paper and PVC board.

SAMPLE REQUIREMENTS
A urine specimen must be collected in a clean and dry container. A
first morning urine specimen is preferred since it generally
contains the highest concentration of HCG, however, urine
specimens collected at any time of the day may be used. Urine
specimens may be stored at 2-8℃ for up to 48 hours prior to assay.
For long-term storage, specimens may be frozen and stored below
-20℃, the frozen specimens should be fully melted and restore to
room temperature and shake before testing .Urine specimens
exhibiting visible precipitates should be centrifuged, filtered, or
allowed to settle to obtain a clear specimen for testing.
Pregnancy Test Kit,HCG Test Strips,HCG Urine Test Kit,HCG Pregnancy Rapid Test Kit
Changchun ZYF science and technology CO.,LTD , https://www.zyf-medical.com