 In 2011, the revenue of China's medical electronics industry was 4 billion U.S. dollars, a year-on-year increase of 19.6%. It is estimated that in 2012 it will increase by 18.4% year-on-year. According to research and analysis, driven by a series of policies, it is expected that the revenue of China's medical electronics industry will almost double in five years from 2011. By 2016, the market will reach 7.4 billion U.S. dollars, and the annual compound annual growth rate will be 13%.
In 2011, the revenue of China's medical electronics industry was 4 billion U.S. dollars, a year-on-year increase of 19.6%. It is estimated that in 2012 it will increase by 18.4% year-on-year. According to research and analysis, driven by a series of policies, it is expected that the revenue of China's medical electronics industry will almost double in five years from 2011. By 2016, the market will reach 7.4 billion U.S. dollars, and the annual compound annual growth rate will be 13%. Specifically, the main policies to be motivated are the "Twelfth Five-Year Plan for Medical Device Technology Industry" published by the Ministry of Science and Technology in early 2012, and the "Health China 2020" strategic study released by the Ministry of Health in August, which states that by 2020, The total expenditure on public health in China will account for 6.5%-7% of GDP. “This size is equivalent to US$63.5 billion. Despite the uncertainties in the economic situation, the Chinese government will continue to increase subsidies to implement relevant policies,†said an expert analysis.
In addition, in response to China's 12th Five-Year Plan, first-tier medical electronics manufacturers plan to introduce cost equipment for second- and third-tier cities. At the same time, domestic manufacturers’ R&D investment is also increasing. Leading domestic manufacturers are realizing differentiated and enhanced brand influence through the introduction of advanced products to change their low-end positioning.
Experts believe that due to the strong demand for personal health monitoring, various types of home-portable medical devices have rapidly become popular products. It is predicted that in 2012, revenue from consumer medical equipment will grow to US$1.1 billion, which is an increase of 19% from 2011.
At the same time, China's consumer medical device market will use semiconductor devices worth approximately US$70 million in 2012, accounting for 28.5% of the total consumer scale. As portability becomes increasingly important for medical devices, vendors are trying to reduce design complexity and the time required to develop finished products.
Pu Zhongjie stated that China's medical device market is still very large. It is also good that some local pharmaceutical companies come in full competition. But first, they must choose the right products for the product. In addition, unlike medical devices and medicines, talents originally engaged in drug research and development in companies do not come to research and develop medical devices. Therefore, it is necessary to reorganize a good team. It is also important to choose a good partner.
It is not easy to form a good team. “Relatively speaking, the marketing team is better established. Pharmaceutical companies can use the original drug marketing channels and talents. It is possible to adapt the medical device market in a short period of time through systematic training.†Jin Kewen pointed out, “But R&D talent is It is a difficult problem that domestic medical device companies have been at a disadvantage in the market competition with multinational medical device companies, largely due to a shortage of talents and a large technological gap, and pharmaceutical companies must move to medical devices in a short time. It is very difficult to develop an excellent R&D team.†This difficulty has been exacerbated by the fact that the R&D forces of more and more multinational medical device companies are moving eastward.
According to media reports, before and after the establishment of an R&D center in China in August, Kehui Medical has dug many R&D talents for a Hong Kong-listed medical device company. Hong Kong-listed medical device companies have no advantage in the fierce competition for talents. How can domestic pharmaceutical companies that have just fought in this field quickly grasp an excellent team?
Industry experts said that only when you go in, you will find many unforeseen problems that have involved production, R&D, sales, and management. In the face of profits and risks, pharmaceutical companies choose to enter or choose not to move. The test is precisely the wisdom and strategy of the company.
Printing Glow In Dark Tape
1. Material and classification of glow in dark tape
We have PET material glow in dark tape and PVC material glow in dark tape. PET material is cheaper but not printable. PVC material support customized printing with low MOQ.
For both materials, we have different kinds according to the glowing time it can last after full charging (0.5hours charging at least). We have 2hours, 4hour, 6hours, 8hours and 10hours according to the glowing period it can last.
2. Colors for choosing
We have light green, pink, orange, red, blue, white for choosing.
3. Features
a. Good ahesion, we use solvent acrylic adhesive, the adhesive is strong and long lasting.
b. Waterproof. Both PET and PVC material are waterproof. Can used for both indoor and outdoor usage.
c. Long service life: 2~3 years even for outdoor usage.
d. Different sizes for choosing: 1.24m x 45.7m log roll, or other customized sizes such as 25mm/ 50mm x 5meters/ 10yards/ 10meters/ 18meters, etc.
e, Accept die cuting to small pieces such as dots, stars, arrows, etc,







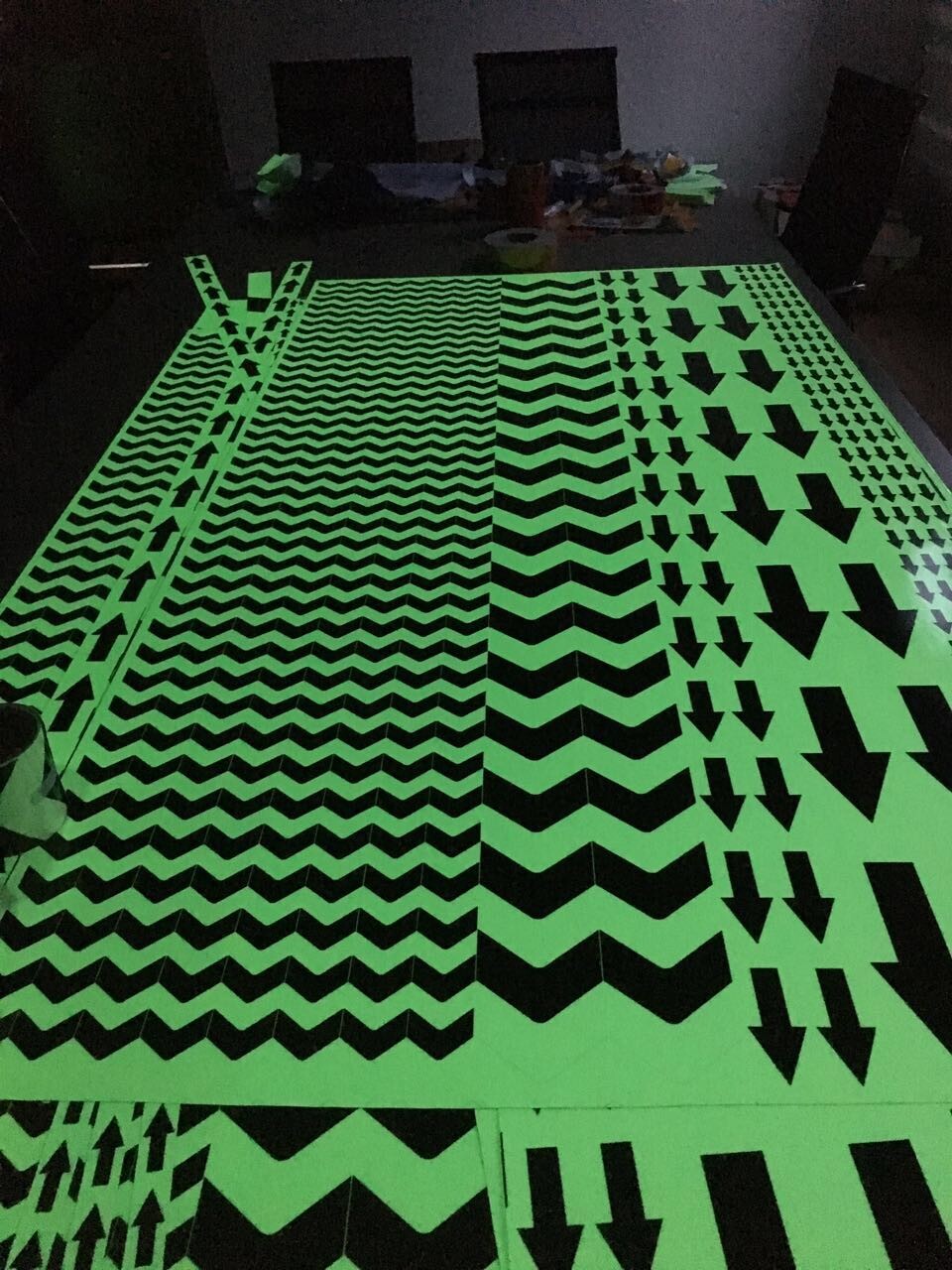
Printing Glowing Tape,Printing Glow In Dark Tape, Customized glow in dark tape, customized luminous tape
Kunshan Jieyudeng Intelligent Technology Co., Ltd. , https://www.jerrytape.com